About Us
CTMC unites robust biopharmaceutical manufacturing with access to MD Anderson Cancer Center’s leading research capabilities into groundbreaking medicines to cure cancer.
WHAT SETS CTMC APART?
Our unique, biotech-friendly model offers more than the standard end-to-end capabilities through our collaborative partnership model. Uniting the strengths of our parent companies, CTMC enables smaller organizations to robustly reach clinical proof of concept and be flexibly positioned for commercialization. From process development to integrated regulatory support, CTMC aims to usher novel cell therapies seamlessly and efficiently through every step of the development journey.
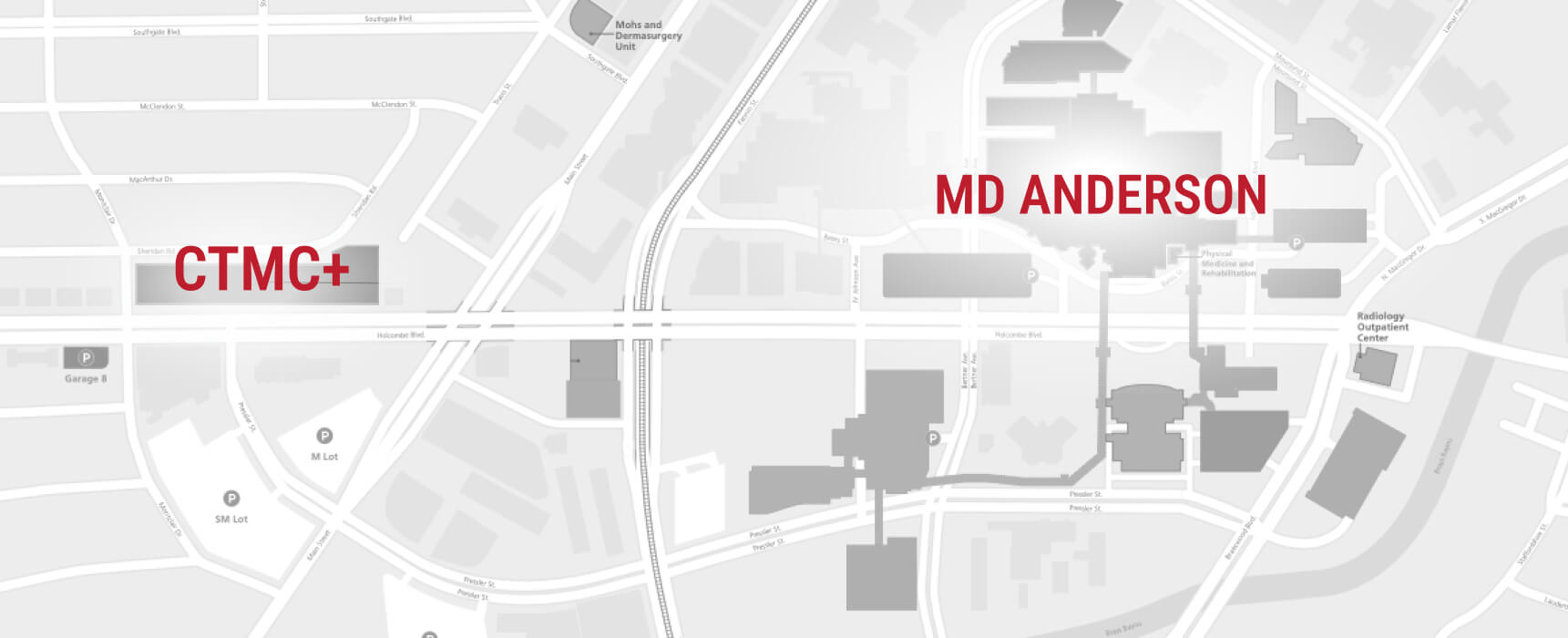
Located in the Texas Medical Center, CTMC’s physical proximity to MD Anderson facilitates collaboration with its expert network of researchers, leverages its robust clinical trial infrastructure, and brings novel, groundbreaking therapies closer to patients.
CTMC is a joint venture between National Resilience and MD Anderson Cancer Center. We were incorporated in May 2022 as a spin-off from the Therapeutics Discovery and Development Division of MD Anderson.
CTMC IS LOCATED WITHIN STEPS OF THE GLOBAL LEADER IN CANCER CARE: MD ANDERSON
Parent Companies
MD Anderson Cancer Center
Resilience

The University of Texas MD Anderson Cancer Center in Houston ranks as one of the world’s most respected centers focused on cancer patient care, research, education, and prevention. The institution's mission is to eliminate cancer in Texas, the nation, and the world.
Founded in 2020, Resilience is building a sustainable network of high-tech, end-to-end manufacturing solutions to ensure the treatments of today and tomorrow can be made quickly, safely, and at scale. By continuously advancing the science of biopharmaceutical manufacturing and development, Resilience seeks to free its partners to focus on the discoveries that improve patients’ lives and protect biopharmaceutical supply chains against future disruptions.
LEADERSHIP / EXECUTIVE TEAM

Jason Bock
Co-Founder + CEO
Jason has over twenty years of research and leadership experience in pharmaceuticals and biotech creating new divisions and companies from the ground up. He sits on several scientific advisory boards. He has a BS in Biology and PhD in Molecular and Cellular Physiology.

Houman Mesghali
Co-Founder + COO
Houman has spent over twenty years building and leading domestic and international teams in both product development and business roles. Houman holds an MSc in Molecular Biology and an MBA.

Priya Balasubramanian
Head of Quality Control + Analytical Development
Priya has vast experience in analytical development supporting the product pipeline from pre-clinical to late-stage cancer therapeutics across multiple modalities including small molecules and cell therapy. She has a B.Tech in Chemical Engineering and a PhD in Chemical and Biomolecular Engineering.
Jason has over twenty years of research and leadership experience in pharmaceuticals and biotech creating new divisions and companies from the ground up. He sits on several scientific advisory boards. He has a BS in Biology and PhD in Molecular and Cellular Physiology.
Houman has spent over twenty years building and leading domestic and international teams in both product development and business roles. Houman holds an MSc in Molecular Biology and an MBA.
Priya has vast experience in analytical development supporting the product pipeline from pre-clinical to late-stage cancer therapeutics across multiple modalities including small molecules and cell therapy. She has a B.Tech in Chemical Engineering and a PhD in Chemical and Biomolecular Engineering.

Chantale Bernatchez
Head of Process Development
Chantale has a PhD in Immunology and more than 20 years of experience in the fields of immunology and T-cell therapy, including academia and biotech work in TIL and CAR-T biology, process development, and manufacturing. She holds four patents on TIL/adoptive cell therapy.

Dan Catron
Head of Corporate Development
Dan has been working in the pharma and biotech sector for more than 15 years focused on aligning therapeutic opportunities with market needs and operating at the intersection of business, law, and science. He has an MSc in Genetics and Genomics and an MBA.

Jane Koo
Head of Regulatory Affairs
Jane has extensive experience working with cross-functional teams to generate effective regulatory strategies and high-quality regulatory submissions. She has provided strategy and documentation for products across all stages of development, from preclinical to commercial. Jane has a BS in Biology and a PhD in Biological Sciences.
Chantale has a PhD in Immunology and more than 20 years of experience in the fields of immunology and T-cell therapy, including academia and biotech work in TIL and CAR-T biology, process development, and manufacturing. She holds four patents on TIL/adoptive cell therapy.
Dan has been working in the pharma and biotech sector for more than 15 years focused on aligning therapeutic opportunities with market needs and operating at the intersection of business, law, and science. He has an MSc in Genetics and Genomics and an MBA.
Jane has extensive experience working with cross-functional teams to generate effective regulatory strategies and high-quality regulatory submissions. She has provided strategy and documentation for products across all stages of development, from preclinical to commercial. Jane has a BS in Biology and a PhD in Biological Sciences.

Laine Linden
Head of Technical Operations
Laine has almost 20 years of experience in the pharmaceutical industry, 13 years of which are directly with Cell Therapy and Viral Vector. Laine holds a BS in Biology.

Jose Torres
Head of Quality Assurance
Jose has more than 30 years of experience in Quality organizations in the pharmaceutical industry, from chemical to gene/cell therapy operations. He has supported several startups and established organizations, developing quality systems and leading quality operations, from clinical to commercial manufacturing, working effectively with cross-functional teams. Jose has a BS in Business Administration.

Erin K. Willert
Head of Project + Alliance Leadership
Erin has more than 20 years of combined research and biotech experience. Erin holds 11 biotherapeutic-related patents and has a BS in Chemistry and a PhD in Biochemistry.
Laine has almost 20 years of experience in the pharmaceutical industry, 13 years of which are directly with Cell Therapy and Viral Vector. Laine holds a BS in Biology.
Jose has more than 30 years of experience in Quality organizations in the pharmaceutical industry, from chemical to gene/cell therapy operations. He has supported several startups and established organizations, developing quality systems and leading quality operations, from clinical to commercial manufacturing, working effectively with cross-functional teams. Jose has a BS in Business Administration.
BOARD OF MANAGERS

Jason Bock, PhD
Co-Founder + CEO
CTMC

Ferran Prat, JD, PhD
Administration + Industry Relations
MD Anderson

Giulio Draetta, MD, PhD
Senior VP + Chief Scientific Officer
MD Anderson

Rahul Singhvi, ScD, MBA
Co-Founder + CEO
Resilience

Ori Solomon, JD
Executive Vice President, Chief Legal + Administrative Officer
Resilience
Jason is the CEO and Co-Founder of CTMC. He has over 20 years of research and leadership experience in pharmaceuticals and biotech, creating new divisions and companies from the ground up. He serves on several scientific advisory boards.
Ferran is Senior VP of Research Administration and Industry Relations at MD Anderson Cancer Center. He leads the institution’s efforts to generate strategic collaborations that bring new drugs, diagnostics, and technologies forward to more quickly benefit patients with cancer.
Giulio is Senior VP and Chief Scientific Officer at MD Anderson Cancer Center. He is Professor and Sewell Distinguished Family Chair in the Department of Genomic Medicine, with a joint appointment in the Department of Molecular and Cellular Oncology.